valence electrons for br|how do you find valence electrons : Baguio Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .
Notable Examples of Internet Chicks Belle Delphine. Belle Delphine is a well-known figure in internet culture for her inventive works that mix makeup, humour, and pictures. Because of her creative and sometimes controversial style, people talk about how online characters become commodities and the limits of internet fame.
PH0 · valence electrons worksheet
PH1 · valence electrons for kids
PH2 · valence electrons chart
PH3 · periodic table valence electrons
PH4 · list of valence electrons for each element
PH5 · how do you find valence electrons
PH6 · finding valence electrons in ions
PH7 · bromine valence electrons
PH8 · Iba pa
Check exact current time in Central Time Zone and discover the key facts: where CT is observed, time change dates, UTC time offset, time zone abbreviations.
valence electrons for br*******There are two ways to find the number of valence electrons in Bromine (Br). The first is to use the Periodic Table to figure out how many electrons Bromine h. Mar 23, 2023 Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the .valence electrons for br how do you find valence electrons Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) . Bromine Valence Electrons. Valence electrons can be found in the p and S highest energy orbitals. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The valence electrons are . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as .
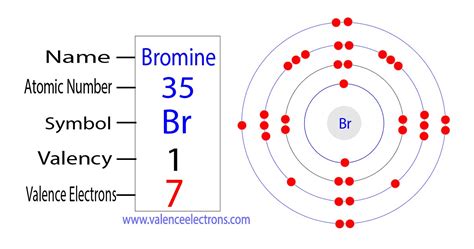
The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine (4s² 3d¹⁰ 4p⁵). Thus, .1.3: Valence electrons and open valences. A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's .
Bromine Valence Electrons. Valence electrons can be found in the p and S highest energy orbitals. Br has an electron configuration of1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. The valence .Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. . CH 3 Br (boiling point 3.5 o C), this has been widely employed to kill pests in the soil, in storage facilities .
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. The \(1s\) electrons in oxygen do not participate in bonding (i.e., chemistry) and are called core electrons. The valence electrons (i.e., the \(2s^22p^4\) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation \(\ref{3}\)), the electrons in the argon-like closed shell are the core . Solution. Steps for Writing Lewis Structures. Example 15.4.1 15.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 2.
Nous pouvons voir que la quantité atomique de brome est de 35 dans les tableaux périodiques. Le nombre total d’électrons dans un atome de brome est donc de 35. Les termes « degré d’oxydation » et « valence » ne sont peut-être pas les mêmes, mais ils sont numériquement presque identiques.
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step. The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.Drawing the Lewis Structure for BrO 3-. Viewing Notes: The BrO 3-Lewis structure has a total of 26 valence electrons. This includes the electron represented by the negative charge in BrO 3-.; You need to put brackets around the BrO 3-Lewis structure as well as a negative charge to show that the structure is a negative ion.; If you calculate the formal .
Microsoft Teams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors.
The atomic number of bromine is 35, which means it has 35 electrons. Now it is possible to find the orbital notation of bromine very easily through electron configuration. That is, the orbital notation of bromine is .valence electrons for br Bromine is a classified halogen and its symbol is ‘Br’. Bromine is the 35th element of the periodic table so its atomic number is 35. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a bromine atom has thirty-five protons and thirty-five electrons. The number of neutrons in an atom .
Total electron pairs are determined by dividing the number total valence electrons by two. For, Br 2 molecule, Total pairs of electrons are seven in their valence shells. Center atom of Br 2 molecule. Because, there are only two atoms and both atoms belongs to same element, do not need to worry about center atom selection. .

When forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. For example, fluorine has seven valence electrons, so it is most .
Method 1: From the Periodic Table. To find out the valence electrons of Bromine, you have to see the position of bromine in the periodic table. More specifically, you have to see the group wise position of Bromine element in the periodic table. From the above image, you can see that the Bromine (Br) is present in the group 17 of periodic table.
Bromine has seven valence electrons, and its valency is one. The given element is bromine and atomic number is 35. The symbol of the bromine element – Br. Electronic configuration: 1s22s22p63s23p64s23d104p5 or [Ar]4s23d104p5. The valency of bromine – +1. Bromine is a chemically reactive metal that is never pure in nature, as it reacts .
Rummy Leader Passion Gaming Reacquires Shares from Rank Group Bobby Garg and Priya Kumar, the co-founders of Indian rummy operator Passion Gaming, have re-acquired all company shares from their investors and are back in control of the company. The company’s shareholders included global gambling giant Rank Group. Rank Group, .
valence electrons for br|how do you find valence electrons